Matter is anything that has mass and volume (it takes up space). This includes most substances we can see and touch, and the particles these are made up of.
It doesn’t include things such as photons – which are particles of light – or waves like those in the electromagnetic spectrum.
That’s because they don’t have mass or volume, so aren't classed as matter.
- States of Matter
Matter can exist in lots of different ways that we call states. Solids, liquids, and gases are the states we are most likely to often see in our everyday lives.
The table below shows the movement of particles and their arrangement (how they are placed) for each state:
State of Matter Image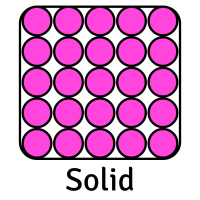 CreditThis work by The Schools' Observatory is licensed under All rights reservedImage
CreditThis work by The Schools' Observatory is licensed under All rights reservedImage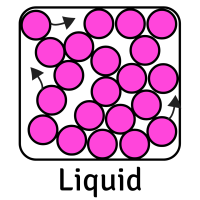 CreditThis work by The Schools' Observatory is licensed under All rights reservedImage
CreditThis work by The Schools' Observatory is licensed under All rights reservedImage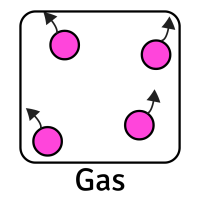 CreditThis work by The Schools' Observatory is licensed under All rights reserved
CreditThis work by The Schools' Observatory is licensed under All rights reservedParticle Arrangement Packed tightly in ordered rows Random (but still touching) with some space between them Random (and not touching) with lots of space between them Particle Movement Vibrate in place Flow past each other Move quickly in random directions Forces between Particles Strong Weaker Weakest This means that each state has certain properties:
Solid Liquid Gas Shape Fixed (stays the same) Changes depending on its container Changes depending on its container Volume Fixed (always takes up same amount of space) Fixed (always takes up same amount of space) Changes to fill its container Can it be compressed (squashed)? No Slightly Yes, very easily Can it be poured? No Yes Yes Whilst we mostly focus on solids, liquids, and gases, there are other states of matter too. Plasma is another example. These are very high temperature and high energy substances with positive and negative charges in them. This creates electric and magnetic fields in the plasma, making it able to conduct electricity.
Most matter in the universe exists as plasma inside stars, nebula, and the space between galaxies. Some examples of plasma on Earth are lightning, the aurora (Northern or Southern lights), and neon lights.
- Changes of State
Matter can change state when it is heated or cooled.
Image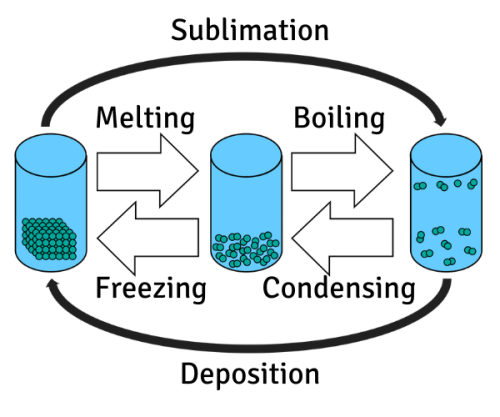 CreditThis work by Enoshd/The Schools' Observatory is licensed under Creative Commons Attribution Share Alike 4.0 International
CreditThis work by Enoshd/The Schools' Observatory is licensed under Creative Commons Attribution Share Alike 4.0 InternationalThe names of the different changes of state Imagine heating up ice. The particles will get energy and start to vibrate faster. This increases the temperature. But since ice is a solid, the particles will stay in ordered rows because of the strong forces between them.
Eventually though, once enough energy has been given, they can overcome these forces a little. At this point, the particles keep moving at the same speed but spread further apart – the substance changes state. Temperature doesn’t change because the movement hasn’t changed, only the arrangement.
The ice has melted into liquid water. If we heated the water, the same thing would happen again. Firstly, the particles move faster and the temperature goes higher. When they have enough energy, their speed will stay the same but they’ll move further apart. The water has boiled to become steam – a gas.
Image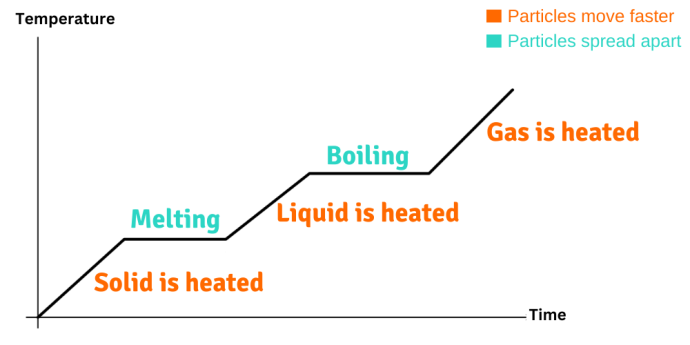 CreditThis work by Bohrium2b/The Schools' Observatory is licensed under Creative Commons Attribution Share Alike 4.0 International
CreditThis work by Bohrium2b/The Schools' Observatory is licensed under Creative Commons Attribution Share Alike 4.0 InternationalA heating curve showing how temperature changes over time. Note that when a change of state happens, the temperature remains the same. The opposite happens when something is cooled down. Energy is taken from the particles, slowing down their movement and decreasing the temperature. When they are moving slowly enough, the forces can pull them closer together. This changes the arrangement of the particles, and a change of state happens.
- Density
- Image
 CreditThis work by Kelvinsong is licensed under Creative Commons Attribution 4.0 International
CreditThis work by Kelvinsong is licensed under Creative Commons Attribution 4.0 InternationalLiquids of different densities Density is how much mass there is in a given volume (or space). The more mass in that space, the greater the density of the substance.
We can calculate density using the equation:
Density = Mass ÷ Volume.
Density is measured in kilograms per metres cubed (kg/m3) or grams per centimetres cubed (g/cm3).
The density of an object can affect whether it will float or sink.
Objects that are denser than water will sink, and those that are less dense will float.
